Consumers
People who want faster clarity and a practical next step when access is limited.
- Smartphone-only experience
- Clear next-step guidance
- Longitudinal photo tracking
From awareness to action
Millions of patients lack timely access to dermatologic evaluation. Primary care shortages and specialist bottlenecks delay diagnosis. SkinAware delivers scalable, smartphone-first triage to help people take the next right step sooner.
Access bottlenecks and long wait times can delay evaluation. That delay drives worse outcomes and avoidable costs.
Many options are not validated, require hardware, or do not fit real care pathways. SkinAware is built for clinically credible triage and follow-through.
Why SkinAware exists, and why the product is built around real access constraints.
A simple workflow from photo to action. Built to integrate into real care pathways.
Take a clear photo with your phone. SkinAware analyzes the image using an ensemble model architecture.
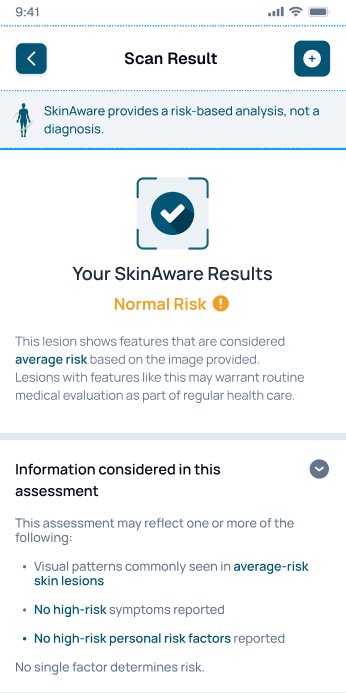
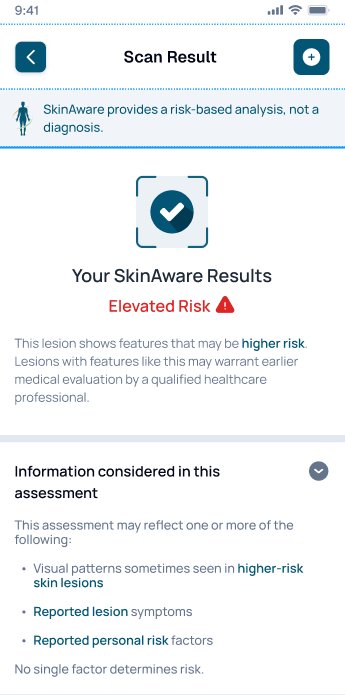
Results are presented as next-step guidance, designed for sensitivity-first triage.
Users can share results with a clinician and access vetted referral options where available.
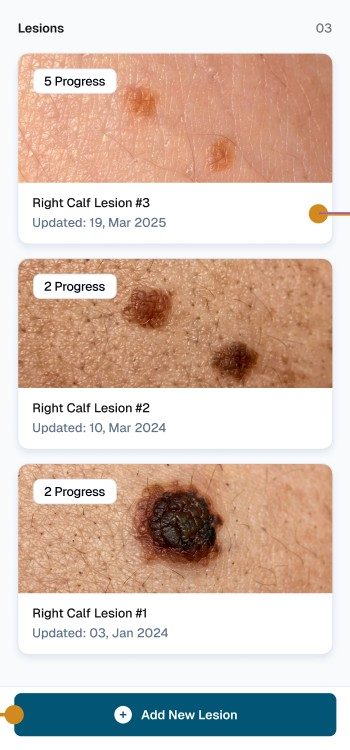
Save photos over time to support monitoring and follow-up conversations with clinicians.
One product narrative that speaks to consumers and validates credibility for researchers and funders.
People who want faster clarity and a practical next step when access is limited.
Partners interested in validation, subgroup performance, and pathway impact.
A scalable access solution with strong unit economics potential and mission alignment.
Internal cross-testing on the ISIC SLICE-3D dataset (217,000+ smartphone-like photos). All Fitzpatrick skin tones included.
Disclosure: Metrics shown are internal benchmark results and are not FDA-cleared claims. They should not be interpreted as diagnostic performance. Clinical validation and prospective trials are planned.
A team with experience in melanoma care, public health, and AI product development.



Clear answers to reduce confusion and manage expectations.
No. SkinAware provides analysis to assist with rapid risk assessment and next-step guidance. It does not diagnose or treat any medical condition.
Seek evaluation by a qualified healthcare professional. If there is bleeding, rapid change, or other concerning symptoms, do not wait for an app result.
Internal benchmarks include all Fitzpatrick skin tones in the test dataset. Ongoing validation is planned to measure and improve subgroup performance.
SkinAware is built as a sensitivity-first triage and referral tool. We plan staged validation and a longer-term FDA path aligned with intended use and claims.
Use the contact form below. We are seeking partners for prospective validation, subgroup performance analysis, and pathway outcome studies.
For pilots, partnerships, research collaborations, or investor updates.